Glycolysis- An over view
- 1. Glycolysis – An overview Biochemistry for Medics www.namrata.co
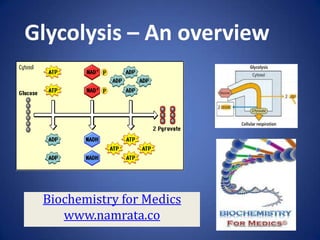
- 2. Glycolysis • Glycolysis is the stepwise degradation of glucose (and other simple sugars). • Glycolysis is a paradigm of metabolic pathways. • Carried out in the cytosol of cells, it is unique, in that it can function either aerobically or anaerobically, depending on the availability of oxygen and the electron transport chain. 1/7/2014 Biochemistry for medics 2
- 3. Overview of Glycolysis • Glycolysis consists of two phases• In the first, a series of five reactions, glucose is broken down to two molecules of glyceraldehyde-3phosphate. • In the second phase, five subsequent reactions convert these two molecules of glyceraldehyde-3phosphate into two molecules of pyruvate. • Phase 1 consumes two molecules of ATP. • The later stages of glycolysis result in the production of four molecules of ATP. • The net is 4 – 2 = 2 molecules of ATP produced per molecule of glucose. 1/7/2014 Biochemistry for medics 3
- 4. Overview of Glycolysis details of oMost of the(the first this pathway metabolic pathway to be elucidated) were worked out in the first half of the 20th century by the German biochemists Otto Warburg, G. Embden, and O. Meyerhof. sequence oIn fact, thein is often of reactions 1/7/2014 Biochemistry for medics referred to as the Embden-Meyerhof pathway. 4
- 5. The First Phase of Glycolysis • Reaction 1: Phosphorylation of Glucose by Hexokinase or Glucokinase —The First Priming Reaction • Glucose enters glycolysis by phosphorylation to glucose 6-phosphate, catalyzed by hexokinase, using ATP as the phosphate donor. • Under physiologic conditions, the phosphorylation of glucose to glucose 6phosphate can be regarded as irreversible. 1/7/2014 Biochemistry for medics 5
- 6. The First Phase of Glycolysis • The formation of such a phosphoester is thermodynamically unfavorable and requires energy input to operate in the forward direction . • The energy comes from ATP, a requirement that at first seems counterproductive. • Glycolysis is designed to make ATP, not consume it. However, the hexokinase, glucokinase reaction is one of two priming reactions in the cycle. 1/7/2014 Biochemistry for medics 6
- 7. Significance of first priming reaction • Phosphorylation keeps the substrate in the cell. Glucose is a neutral molecule and could diffuse across the cell membrane, but phosphorylation confers a negative charge on glucose, and the plasma membrane is essentially impermeable to glucose-6-phosphate • Rapid conversion of glucose to glucose-6phosphate keeps the intracellular concentration of glucose low, favoring diffusion of glucose into the cell. 1/7/2014 Biochemistry for medics 7
- 8. Significance of first priming reaction • The addition of the phosphoryl group begins to destabilize glucose, thus facilitating its further metabolism. • Further more, because regulatory control can be imposed only on reactions not at equilibrium, the favorable thermodynamics of this first reaction makes it an important site for regulation. 1/7/2014 Biochemistry for medics 8
- 9. Significance of first priming reaction • Phosphorylation of glucose to glucose-6-phosphate by ATP creates a charged molecule that cannot easily cross the plasma membrane. 1/7/2014 Biochemistry for medics 9
- 10. First priming reaction • In most animal, plant, and microbial cells, the enzyme that phosphorylates glucose is hexokinase. • Magnesium ion (Mg2+) is required for this reaction • Hexokinase can phosphorylate a variety of hexose sugars, including glucose, mannose, and fructose. • Hexokinase reacts strongly with glucose, while its affinity for fructose and galactose is relatively low. 1/7/2014 Biochemistry for medics 10
- 11. First priming reaction • Hexokinase (contd) • The enzyme is allosterically inhibited by the product, glucose-6-phosphate. • The hexokinase reaction is one of three points in the glycolysis pathway that are regulated. • The apparent Km for glucose of the enzyme is approximately 0.05 mM/L, and the enzyme thus operates efficiently at normal blood glucose levels of 4 mM. • Different body tissues possess different isozymes of hexokinase, each exhibiting somewhat different kinetic properties. 1/7/2014 Biochemistry for medics 11
- 12. First priming reaction • Glucokinase occurs in cells in the liver, pancreas, gut, and brain of humans and most other vertebrates. • In each of these organs it plays an important role in the regulation of carbohydrate metabolism by acting as a glucose sensor, triggering shifts in metabolism or cell function in response to rising or falling levels of glucose, such as occur after a meal or when fasting. • Mutations of the gene for this enzyme can cause unusual forms of diabetes or hypoglycemia. 1/7/2014 Biochemistry for medics 12
- 13. Hexokinase versus Glucokinase Characteristics Hexokinase Glucokinase Tissue distribution: Most tissues Liver and β cells of Pancreas Km Low (0.05 mM/L) High (10 mM/L) Vmax Low High Inhibition by G6P Yes No Inducible No Inducible(the amount present in the liver is controlled by insulin) Clinical significance Deficiency causes hemolytic anemia Patients with diabetes mellitus show less activity Biological Significance Involved in maintaining intracellular glucose concentration Involved in maintaining blood glucose concentration 1/7/2014 Biochemistry for medics 13
- 14. Glucokinase versus Glucose-6Phosphatase In the liver, the action of Glucokinase is opposed by the action of glucose-6-phosphatase. The balance between glucokinase and glucose-6phosphatase slides back and forth, increasing uptake to the liver and phosphorylation when the level of blood glucose is high, and releasing glucose from G6-P when blood glucose falls. The function of glucokinase in the liver is to remove glucose from the blood following a meal, providing glucose 6-phosphate in excess of requirements for glycolysis, which is used for glycogen synthesis and lipogenesis. 1/7/2014 Biochemistry for medics 14
- 15. Fate of Glucose-6-P Glucose 6-phosphate is an important compound at the junction of several metabolic pathways: Glycolysis Gluconeogenesis Pentose phosphate pathway, Glycogenesis, and Glycogenolysis. 1/7/2014 Biochemistry for medics 15
- 16. Reaction 2: Isomerization of Glucose-6Phosphate to Fructose-6-Phosphate • This amounts to isomerization of an aldose (glucose-6phosphate) to a ketose—fructose-6-phosphate • The reaction is catalyzed by Phospho gluco isomerase • The reaction is necessary for two reasons. o First, the next step in glycolysis is phosphorylation at C-1, and the hemiacetal -OH of glucose would be more difficult to phosphorylate than a simple primary hydroxyl . o Second, the isomerization to fructose (with a carbonyl group at position 2 in the linear form) activates carbon C-3 for cleavage in the fourth step of glycolysis. 1/7/2014 Biochemistry for medics 16
- 17. Reaction catalyzed by Phosphoglucose isomerase The carbonyl oxygen of glucose-6-phosphate is shifted from C-1 to C-2. 1/7/2014 Biochemistry for medics 17
- 18. Reaction 3: Phospho fructokinase - The Second Priming Reaction • The action of Phosphoglucoisomerase, “moving” the carbonyl group from C-1 to C-2, creates a new primary alcohol function at C-1 • The next step in the glycolytic pathway is the phosphorylation of this group by phosphofructo kinase. • Phosphofructo kinase reaction commits the cell to metabolizing glucose rather than converting it to another sugar or storing it. 1/7/2014 Biochemistry for medics 18
- 19. Reaction 3: Phospho fructokinase The Second Priming Reaction The phosphofructo kinase reaction is an important site of regulation— indeed, the most important site in the glycolytic pathway. 1/7/2014 Biochemistry for medics 19
- 20. Reaction 4: Cleavage of Fructose-1,6Bisphosphate Fructose bisphosphate aldolase cleaves fructose-1,6bisphosphate between the C-3 and C-4 carbons to yield two triose phosphates. The products are Dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3phosphate. 1/7/2014 Biochemistry for medics 20
- 21. Reaction 5: Triose Phosphate Isomerase • Of the two products of the Aldolase reaction, only glyceraldehyde-3phosphate goes directly into the second phase of glycolysis. • The other triose phosphate, Dihydroxyacetone phosphate, must be converted to glyceraldehyde-3phosphate by the enzyme triose phosphate Isomerase. 1/7/2014 Biochemistry for medics 21
- 22. Reaction catalyzed by Triose Phosphate Isomerase • This reaction thus permits both products of the aldolase reaction to continue in the glycolytic pathway • The triose phosphate Isomerase reaction completes the first phase of glycolysis, each glucose molecule that passes through being converted to two molecules of glyceraldehyde-3-phosphate. for medics 1/7/2014 Biochemistry 22
- 23. The Second Phase of Glycolysis • The second half of the glycolytic pathway involves the reactions that convert the metabolic energy in the glucose molecule into ATP. • Reaction 6: Glyceraldehyde-3-Phosphate Dehydrogenase • The enzyme catalyzing this oxidation, glyceraldehyde 3-phosphate dehydrogenase, is NAD+dependent 1/7/2014 Biochemistry for medics 23
- 24. Reaction catalyzed by Glyceraldehyde-3-P dehydrogenase Glyceraldehyde-3-P dehydrogenase catalyzes the formation of a high energy compound. This is the first step in the payoff phase. 1/7/2014 Biochemistry for medics 24
- 25. Reaction 6: Glyceraldehyde-3-Phosphate Dehydrogenase (contd.) • Four —SH groups are present on each polypeptide, derived from cysteine residues within the polypeptide chain. • One of the —SH groups is found at the active site of the enzyme . • The substrate initially combines with this —SH group, forming a thio hemiacetal that is oxidized to a thiol ester; the hydrogens removed in this oxidation are transferred to NAD+. 1/7/2014 Biochemistry for medics 25
- 26. Reaction 6: Glyceraldehyde-3-Phosphate Dehydrogenase (contd.) • The enzyme can be inactivated by reaction with iodoacetate, which reacts with and blocks the essential cysteine sulfhydryl. • The glyceraldehyde-3-phosphate dehydrogenase reaction is the site of action of arsenate (AsO43-), an anion analogous to phosphate. • Arsenate is an effective substrate in this reaction, forming 1-arseno-3-phosphoglycerate, but acyl arsenates are quite unstable and are rapidly hydrolyzed. 1-Arseno-3-phosphoglycerate breaks down to yield 3-phosphoglycerate, the product of the seventh reaction of glycolysis. 1/7/2014 Biochemistry for medics 26
- 27. Reaction 7 : Phosphoglycerate Kinase • The enzyme phosphoglycerate kinase transfers a phosphoryl group from 1,3bisphosphoglycerate to ADP to form an ATP • Because each glucose molecule sends two molecules of glyceraldehyde-3-phosphate into the second phase of glycolysis and because two ATPs were consumed per glucose in the firstphase reactions, the phosphoglycerate kinase reaction “pays off” the ATP debt created by the priming reactions. 1/7/2014 Biochemistry for medics 27
- 28. Reaction catalyzed by phospho glycerate kinase The enzyme phosphoglycerate kinase transfers the high-energy phosphate group from the carboxyl group of 1,3bisphosphoglycerate to ADP, forming ATP and 3-phosphoglycerate 1/7/2014 Biochemistry for medics 28
- 29. Reaction 7 : Phosphoglycerate Kinase (contd.) • ADP is phosphorylated to form ATP at the expense of a substrate, namely, glyceraldehyde-3-phosphate. This is an example of substrate-level phosphorylation. • In the presence of Arsenate the molecule of ATP formed in reaction 7 ( phosphoglycerate kinase ) is not made because this step has been bypassed. • The lability of 1-arseno-3-phosphoglycerate effectively uncouples the oxidation and phosphorylation events, which are normally tightly coupled in the glyceraldehyde-3-phosphate dehydrogenase reaction. 1/7/2014 Biochemistry for medics 29
- 30. Rapapport Luebering- shunt (R.L. Shunt ) • An important regulatory molecule, 2,3bisphosphoglycerate, is synthesized and metabolized by a pair of reactions that make a detour around the phosphoglycerate kinase reaction. • 2,3-BPG, which stabilizes the deoxy form of hemoglobin and is primarily responsible for the cooperative nature of oxygen binding by hemoglobin, is formed from 1,3bisphosphoglycerate by bisphosphoglycerate mutase 1/7/2014 Biochemistry for medics 30
- 31. R.L. Shunt Hydrolysis of 2,3-BPG is carried out by 2,3-bisphosphoglycerate phosphatase . Although other cells contain only a trace of 2,3-BPG, erythrocytes typically 1/7/2014 contain 4 to 5 mM 2,3-BPG. Biochemistry for medics 31
- 32. Significance of 2,3-bisphosphoglycerate a) Unloading of Oxygen o When 2,3-BPG binds to deoxyhemoglobin, it acts to stabilize the low oxygen affinity state (T state) of the oxygen carrier o By selectively binding to deoxyhemoglobin, 2,3-BPG stabilizes the T state conformation, making it harder for oxygen to bind hemoglobin and more likely to be released to adjacent tissues. 1/7/2014 Biochemistry for medics 32
- 33. Significance of 2,3-bisphosphoglycerate (contd.) • b) Effect of Hypoxia • 2,3-BPG can help to prevent tissue hypoxia in conditions where it is most likely to occur. • Conditions of low tissue oxygen concentration such as high altitude (2,3-BPG levels are higher in those acclimated to high altitudes), airway obstruction, anemias or congestive heart failure will tend to cause RBCs to generate more 2,3-BPG in their effort to generate energy by allowing more oxygen to be released in tissues deprived of oxygen. • This release is potentiated by the Bohr effect in tissues with high energetic demands. 1/7/2014 Biochemistry for medics 33
- 34. Significance of 2,3-bisphosphoglycerate (contd.) Effect of Binding of 2,3 BPG to hemoglobin 1/7/2014 Biochemistry for medics 34
- 35. Significance of 2,3-bisphosphoglycerate (contd.) d) Fetal hemoglobin (HbF) and 2,3 BPG o Fetal hemoglobin (HbF) exhibits a low affinity for 2,3BPG, resulting in a higher binding affinity for oxygen. o This increased oxygen-binding affinity relative to that of adult hemoglobin (HbA) is due to HbF's having two α/γ dimers as opposed to the two α/β dimers of HbA. o The positive histidine residues of HbA β-subunits that are essential for forming the 2,3-BPG binding pocket are replaced by serine residues in HbF γ-subunits. o 2,3-BPG has difficulties in linking to the fetal hemoglobin, so the affinity of fetal hemoglobin for O2 increases . o That’s the way O2 flows from the mother to the fetus. 1/7/2014 Biochemistry for medics 35
- 36. Reaction 8: Phosphoglycerate Mutase • The remaining steps in the glycolytic pathway prepare for synthesis of the second ATP equivalent. • This begins with the phosphoglycerate mutase reaction in which the phosphoryl group of 3-phosphoglycerate is moved from C-3 to C-2. 1/7/2014 Biochemistry for medics 36
- 37. Reaction 8: Phosphoglycerate Mutase (contd.) The term mutase is applied to enzymes that catalyze migration of a functional group within a substrate molecule. 1/7/2014 Biochemistry for medics 37
- 38. Reaction 9: Enolase o This reaction of glycolysis makes a highenergy phosphate in preparation for ATP synthesis. o Enolase catalyzes the formation of phosphoenolpyruvate from 2phosphoglycerate . o The reaction in essence involves a dehydration—the removal of a water molecule—to form the enol structure of PEP. 1/7/2014 Biochemistry for medics 38
- 39. Reaction 9: Enolase • The enzyme is strongly inhibited by fluoride ion in the presence of phosphate. • Inhibition arises from the formation of fluorophosphate (FPO32-), which forms a complex with Mg2+ at the active site of the enzyme. 1/7/2014 Biochemistry for medics 39
- 40. Reaction 10: Pyruvate Kinase • The second ATP-synthesizing reaction of glycolysis is catalyzed by pyruvate kinase, which brings the pathway at last to its pyruvate branch point. • Pyruvate kinase mediates the transfer of a phosphoryl group from phosphoenolpyruvate to ADP to make ATP and pyruvate . 1/7/2014 Biochemistry for medics 40
- 41. Reaction 10: Pyruvate Kinase (contd.) The reaction requires Mg2+ ion and is stimulated by K+ and certain other monovalent cations 1/7/2014 Biochemistry for medics 41
- 42. Reaction 10: Pyruvate Kinase (contd.) • For each glucose molecule in the glycolysis pathway, two ATPs are made at the pyruvate kinase stage (because two triose molecules were produced per glucose in the aldolase reaction). • Because the pathway broke even in terms of ATP at the phosphoglycerate kinase reaction (two ATPs consumed and two ATPs produced), the two ATPs produced by pyruvate kinase represent the “payoff” of glycolysis —a net yield of two ATP molecules. 1/7/2014 Biochemistry for medics 42
- 43. Formation of keto form of Pyruvate The enol form tautomerizes rapidly and nonenzymatically to yield the keto form of pyruvate, the form that predominates at pH 7. 1/7/2014 Biochemistry for medics 43
- 44. The Metabolic Fates of NADH and Pyruvate —The Products of Glycolysis • In addition to ATP, the products of glycolysis are NADH and pyruvate. • Their processing depends upon other cellular pathways. • NADH must be recycled to NAD+, lest NAD+ become limiting in glycolysis. • NADH can be recycled by both aerobic and anaerobic paths, either of which results in further metabolism of pyruvate. • What a given cell does with the pyruvate produced in glycolysis depends in part on the availability of oxygen 1/7/2014 Biochemistry for medics 44
- 45. The Metabolic Fates of NADH and Pyruvate —The Products of Glycolysis • Under aerobic conditions, pyruvate can be sent into the citric acid cycle, where it is oxidized to CO2 with the production of additional NADH (and FADH2). • Under aerobic conditions, the NADH produced in glycolysis and the citric acid cycle is reoxidized to NAD+ in the mitochondrial electron transport chain. 1/7/2014 Biochemistry for medics 45
- 46. The Metabolic Fates of NADH and Pyruvate —The Products of Glycolysis • Under anaerobic conditions, the NADH cannot be reoxidized through the respiratory chain to oxygen. • Pyruvate is reduced by the NADH to lactate, catalyzed by lactate dehydrogenase. • There are different tissue specific isoenzymes lactate dehydrogenases that have clinical significance. • The reoxidation of NADH via lactate formation allows glycolysis to proceed in the absence of oxygen by regenerating sufficient NAD+ for another cycle of the reaction catalyzed by glyceraldehyde-3phosphate dehydrogenase. 1/7/2014 Biochemistry for medics 46
- 47. Coupling of reactions 1/7/2014 Biochemistry for medics 47
- 48. Product of glycolysis under anaerobic conditions • Tissues that function under Hypoxic Conditions Produce Lactate • This is true of skeletal muscle, particularly the white fibers • Glycolysis in erythrocytes always terminates in lactate, because the subsequent reactions of pyruvate oxidation are mitochondrial, and erythrocytes lack mitochondria. • Other tissues that normally derive much of their energy from glycolysis and produce lactate include brain, gastrointestinal tract, renal medulla, retina, and skin. • The liver, kidneys, and heart usually take up lactate and oxidize it but will produce it under hypoxic conditions. 1/7/2014 Biochemistry for medics 48
- 49. Energy yield per molecule of Glucose oxidized through Glycolysis S. No. Reaction catalyzed Mode of ATP formation ATP per molecule of Glucose 1. Glyceraldehyde 3-phosphate dehydrogenase Respiratory chain 6 oxidation of 2 NADH 2. Phosphoglycerate kinase Substrate level phosphorylation 2 3. Pyruvate kinase Substrate level phosphorylation 2 4. Consumption of ATP for reactions of hexokinase and phosphofructo kinase -2 5. Net ATP yield 8 Under anaerobic conditions Electron transport chain does not operate so the ATP is only formed by substrate level phosphorylation. Hence the total 1/7/2014 energy yield through glycolysis in the absence of oxygen is only 2 Biochemistry for medics 49 ATP per Mol of Glucose.
- 50. Steps of Glycolysis 1/7/2014 Biochemistry for medics 50
- 51. Regulation of Glycolysis Flux through a metabolic pathway can be regulated in several ways: 1. Availability of substrate 2. Concentration of enzymes responsible for rate-limiting steps 3. Allosteric regulation of enzymes 4. Covalent modification of enzymes (e.g. phosphorylation) 1/7/2014 Biochemistry for medics 51
- 52. Regulation of Glycolysis (contd.) • Enzymes that catalyze 3 irreversible steps in glycolytic pathways are potential sites for regulatory control. • The enzymes responsible for catalyzing these three steps, hexokinase (or glucokinase) for step 1, phosphofructo kinase for step 3, and pyruvate kinase for step 10, are the primary steps for allosteric enzyme regulation. • Availability of substrate (in this case, glucose), is another general point for regulation. 1/7/2014 Biochemistry for medics 52
- 53. Regulation of Glycolysis (contd.) • The concentration of these three enzymes in the cell is regulated by hormones that affect their rates of transcription. • Insulin upregulates the transcription of Glucokinase, phosphofructo kinase, and pyruvate kinase, while glucagon down regulates their transcription. • These effects take place over a period of hours to days, and generally reflect whether a person is well-fed or starving. 1/7/2014 Biochemistry for medics 53
- 54. Regulation of Glycolysis (contd.) 1) Regulation at the level of Hexokinase and Glucokinase • The Hexokinase enzyme is allosterically inhibited by the product, glucose-6-phosphate. • Glucokinase is highly specific for D-glucose, has a much higher Km for glucose (approximately 10.0 mM ), and is not product-inhibited. • With such a high Km for glucose, Glucokinase becomes important metabolically only when liver glucose levels are high. • Glucokinase is an inducible enzyme—the amount present in the liver is controlled by insulin. 1/7/2014 Biochemistry for medics 54
- 55. Regulation of Glycolysis (contd.) 2) Regulation of Phospho fructokinase a) Role of ATP- ATP is an allosteric inhibitor of this enzyme. • In the presence of high ATP concentrations, the Km for fructose-6-phosphate is increased, glycolysis thus “turns off. • AMP reverses the inhibitory action of ATP, and so the activity of the enzyme increases when the ATP/AMP ratio is lowered. In other words, glycolysis is stimulated as the energy charge falls. 1/7/2014 Biochemistry for medics 55
- 56. Regulation of Glycolysis (contd.) 2) Regulation of Phospho fructokinase b) Role of Citrate o Phosphofructokinase is inhibited by citrate, an early intermediate in the citric acid cycle. o A high level of citrate means that biosynthetic precursors are abundant and additional glucose should not be degraded for this purpose. o Citrate inhibits phosphofructokinase by enhancing the inhibitory effect of ATP 1/7/2014 Biochemistry for medics 56
- 57. Regulation of Glycolysis (contd.) 2) Regulation of Phospho fructokinase c) Role of Fr 2,6 bisphosphate o Phosphofructokinase is also regulated by Dfructose-2,6-bisphosphate, a potent allosteric activator that increases the affinity of phosphofructokinase for the substrate fructose-6phosphate o Fructose-2,6-bisphosphate increases the net flow of glucose through glycolysis by stimulating phosphofructokinase and, by inhibiting fructose-1,6bisphosphatase, the enzyme that catalyzes this reaction in the opposite direction. 1/7/2014 Biochemistry for medics 57
- 58. Regulation of Glycolysis (contd.) Role of Fr 2,6 bisphosphate 1/7/2014 Biochemistry for medics 58
- 59. Regulation of Glycolysis (contd.) Why is phosphofructokinase rather than hexokinase the pacemaker of glycolysis? o Glucose 6-phosphate is not solely a glycolytic intermediate. o Glucose 6-phosphate can also be converted into glycogen or it can be oxidized by the pentose phosphate pathway to form NADPH. o The first irreversible reaction unique to the glycolytic pathway, the committed step, is the phosphorylation of fructose 6- phosphate to fructose 1,6-bisphosphate. o Thus, it is highly appropriate for phosphofructokinase to be the primary control site in glycolysis. 1/7/2014 Biochemistry for medics 59
- 60. Regulation of Glycolysis (contd.) 3) Regulation of pyruvate Kinase o It is activated by AMP and fructose-1,6-bisphosphate and inhibited by ATP, acetyl-CoA, and alanine. • Liver pyruvate kinase is regulated by covalent modification. • Hormones such as glucagon activate a cAMPdependent protein kinase, which transfers a phosphoryl group from ATP to the enzyme. • The phosphorylated form of pyruvate kinase is more strongly inhibited by ATP and alanine and has a higher Km for PEP, so that, in the presence of physiological levels of PEP, the enzyme is inactive. 1/7/2014 Biochemistry for medics 60
- 61. Regulation of Glycolysis (contd.) This hormone-triggered phosphorylation, prevents the liver from consuming glucose when it is more urgently needed by brain and muscles. 1/7/2014 Biochemistry for medics 61
- 62. Inhibitors of Glycolysis a) Arsenate and Iodoacetate- Inhibitors of Glyceraldehyde-3-P dehydrogenase b) Bromo hydroxy acetone phosphateInhibitor of dihydroxy acetone phosphate c) Fluoride- Inhibitor of Enolase d) Oxamate- Inhibitor of Lactate dehydrogenase 1/7/2014 Biochemistry for medics 62
- 63. Significance of glycolysis other than energy production • Glucose-6-P is a common intermediate for a number of pathways and is used depending on the need of the cell, like glycogen synthesis, Uronic acid pathway, HMP pathway etc. • Fructose-6-P is used for the synthesis of Glucosamines. • Triose like glyceraldehyde-3-P and other glycolytic intermediates can be used in the HMP pathway for the production of pentoses. • Dihydroxy Acetone –phosphate can be used for the synthesis of Glycerol -3-P , which is used for the synthesis of Triglycerides or phospholipids. 1/7/2014 Biochemistry for medics 63
- 64. Significance of glycolysis other than energy production (contd.) • 2,3 BPG is an important compound produced pathway in erythrocytes in the glycolytic pathway for unloading of O2 to the peripheral tissues. • The sugars like Fructose, Galactose. Mannose and even Glycerol can be oxidized in glycolysis. • Out of the total 10 reactions of Glycolysis, 7 reactions are reversible and are used for the synthesis of Glucose by the process of Gluconeogenesis. • Pyruvate the end product of glycolysis provides precursor for the TCA cycle and for the synthesis of other compounds. 1/7/2014 Biochemistry for medics 64
- 65. Clinical significance Pyruvate kinase deficiency • Manifested by hemolytic anemia Biochemical basis for hemolytic anemia Pyruvate kinase activity is critical for the pathway and therefore critical for energy production. • If ATP is not produced in amounts sufficient to meet the energy demand, then those functions are compromised. • Energy is required to maintain the Na+/K+ balance within the RBC and to maintain the flexible discoid shape of the cell. • In the absence of sufficient pyruvate kinase activity and therefore ATP, the ionic balance fails, and the membrane becomes misshapen. 1/7/2014 Biochemistry for medics 65
- 66. Clinical significance Pyruvate kinase deficiency(contd.) o Important intermediates proximal to the PK defect influence erythrocyte function. o Two- to 3-fold increases of 2, 3-bisphosphoglycerate levels result in a significant rightward shift in the hemoglobin-oxygen dissociation curve. o Physiologically, the hemoglobin of affected individuals has an increased capacity to release oxygen into the tissues, thereby enhancing oxygen delivery. o Thus, for a comparative hemoglobin and Haemtocrit level, an individual with PKD has an enhanced exercise capacity and fewer symptoms. 1/7/2014 Biochemistry for medics 66
- 67. Substrates other than glucose used in Glycolysis o The sugars like Fructose, Galactose. Mannose and even Glycerol produced from hydrolysis of triglycerides or obtained from diet or other sources can be oxidized through glycolysis. 1/7/2014 Biochemistry for medics 67
- 68. Why is glucose instead of some other monosaccharide such a prominent fuel? • • • • • First, glucose is one of the monosaccharides formed from formaldehyde under prebiotic conditions, so it may have been available as a fuel source for primitive biochemical systems. Second, glucose has a low tendency, relative to other monosaccharides, to nonenzymatically glycosylate proteins. In their open-chain (carbonyl) forms, monosaccharides can react with the amino groups of proteins to form Schiff bases, which rearrange to form a more stable amino ketone linkage. Such nonspecifically modified proteins often do not function effectively. Glucose has a strong tendency to exist in the ring formation and, consequently, relatively little tendency to modify proteins. 1/7/2014 Biochemistry for medics 68
- 69. For further details http://www.namrata.co/glycolysis-lecture-1/ http://www.namrata.co/glycolysis-lecture-2/ http://www.namrata.co/glycolysis-lecture-3/ http://www.namrata.co/regulation-ofglycolysis/ • http://www.namrata.co/glycolysis-subjectivequestions-solved/ • • • • 1/7/2014 Biochemistry for medics 69
